Please note that the end station described below, and the 1 kHz XUV beamline, will soon be upgraded as part of the “HiLUX” upgrade project. When these upgrades become available for user access, this section will be updated accordingly. You can read more about the upgrade project at: https://www.clf.stfc.ac.uk/Pages/HiLUX.aspx.
Gas-phase AMO end-station
The AMO end-station (Fig. 2) comprises two coupled ultra-high vacuum chambers, pumped using a 3200 L/s turbomolecular vacuum pump. The lower chamber houses the gas source, using either a pulsed gas nozzle from MassSpec BV or a continuous effusive nozzle. The molecular beams produced by either of these sources pass through a skimmer to enter the second chamber, the reaction chamber. The laser pulses generated by our 1 kHz beamline interact with the molecular beam within an ion optic stack housed in the reaction chamber, generating photoelectrons and photoions that are measured using a Kaesdorf ETF11 time-of-flight spectrometer or via velocity map imaging.
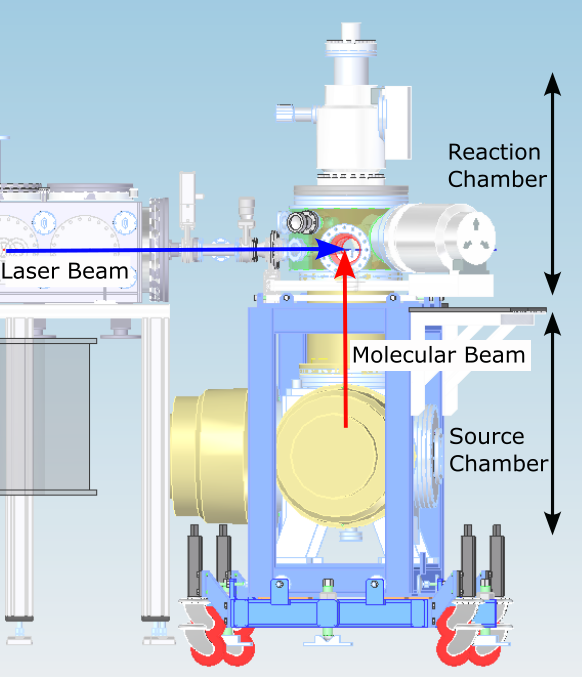
Fig. 2: Schematic of the AMO end-station: a source chamber produces the molecular beam, which interacts with the laser beam within the reaction chamber.
Beamline Capabilities
The Artemis AMO end-station is used with our 1 kHz laser system and XUV beamline. More information can be found at the following links:
In short, the AMO end-station can study photochemical reactions induced by IR and UV tunable wavelength laser sources, or by XUV laser pulses generated by high harmonic generation (HHG) in an Argon gas jet. The parameters listed below give a general description of the capabilities of the 1 kHz beamline and serve as a starting point for discussions with the beamline scientists, who can advise on the best arrangements for the particular needs of your experiment.
| Tunable Ti:Sapphire CPA OPA laser system
| XUV HHG beamline
|
Wavelengths
| 235 nm - 15 um (tunable)
| ~30 nm – 80 nm (15 eV – 45 eV)
|
Pulse duration
| 30 fs
| ~ 30 fs
|
Polarisation
| Fully controllable
| Linear polarisation, s or p
|
Flux
| | ~ 2x1010 photons/second at 30 eV
|
Pulse energy
| Pulse energies in excess of 100 uJ at 250 nm and 2 uJ
at 20 um can be obtained.
| |
Technical notes for preparing a user proposal
Sample: Please consider and include conditions of the molecular beam required for your experiment when preparing a user proposal (e.g., do you require heating, cooling, seeding). If seeding in another gas is required, please consider whether the ionisation potential of the seed gas overlaps significantly with that of the photochemical system or processes you wish to study.
Spectrometer: Include description of the experimental regime to be used (i.e. measurement of electrons or ions under time-of-flight or VMI conditions) so that the correct spectrometer configuration can be implemented ahead of the beamtime.
Laser requirements: Include characteristics of pump and probe laser pulses you wish to use in the experiment (wavelengths, pulse durations, energy per pulse, polarisation). The XUV laser beam is generated by high harmonic generation in a gas jet. Please specify the required XUV photon energy in your proposal so that this can be optimised ahead of the beamtime. Please communicate with beamline staff before you submit your proposal to confirm that your laser requirements are achievable.
Timescale: Take into consideration time required to perform pump-probe acquisitions. The time taken for an acquisition will depend on fluence limitations, and the statistics of the data acquired at each delay point, and the number of delay points recorded over the expected time scale of the phenomena of interest.
References
[1]
Smith, Adam D., Emily M. Warne, Darren Bellshaw, Daniel A. Horke, Maria Tudorovskya, Emma Springate, Alfred J. H. Jones, et al.
‘Mapping the Complete Reaction Path of a Complex Photochemical Reaction’. Physical Review Letters
120, no. 18 (4 May 2018): 183003.
https://doi.org/10.1103/PhysRevLett.120.183003.
[2]
Warne, Emily M., Briony Downes-Ward, Joanne Woodhouse, Michael A. Parkes, Emma Springate, Philip A. J. Pearcy, Yu Zhang, et al. ‘
Photodissociation Dynamics of Methyl Iodide Probed Using Femtosecond Extreme Ultraviolet Photoelectron Spectroscopy’. Physical Chemistry Chemical Physics
22, no. 44 (2020): 25695–703.
https://doi.org/10.1039/D0CP03478A.
[3]
Warne, Emily M., Adam D. Smith, Daniel A. Horke, Emma Springate, Alfred J. H. Jones, Cephise Cacho, Richard T. Chapman, and Russell S. Minns. ‘
Time Resolved Detection of the S(1D) Product of the UV Induced Dissociation of CS2’. The Journal of Chemical Physics
154, no. 3 (21 January 2021): 034302.
https://doi.org/10.1063/5.0035045.
[4]
Galinis, Gediminas, Cephise Cacho, Richard T. Chapman, Andrew M. Ellis, Marius Lewerenz, Luis G. Mendoza Luna, Russell S. Minns, et al.
‘Probing the Structure and Dynamics of Molecular Clusters Using Rotational Wave Packets’. Physical Review Letters
113, no. 4 (24 July 2014): 043004.
https://doi.org/10.1103/PhysRevLett.113.043004
[5]
Lee, Jason W. L., Hansjochen Köckert, David Heathcote, Divya Popat, Richard T. Chapman, Gabriel Karras, Paulina Majchrzak, Emma Springate, and Claire Vallance. ‘
Three-Dimensional Covariance-Map Imaging of Molecular Structure and Dynamics on the Ultrafast Timescale’. Communications Chemistry
3, no. 1 (December 2020): 72.
https://doi.org/10.1038/s42004-020-0320-3
[6]
Ganjitabar, Hassan, Dhirendra P. Singh, Richard Chapman, Adrian Gardner, Russell S. Minns, Ivan Powis, Katharine L. Reid, and Arno Vredenborg. ‘
The Role of the Intermediate State in Angle-Resolved Photoelectron Studies Using (2 + 1) Resonance-Enhanced Multiphoton Ionization of the Chiral Terpenes, α-Pinene and 3-Carene’. Molecular Physics
119, no. 1–2 (17 January 2021): e1808907.
https://doi.org/10.1080/00268976.2020.1808907.
