The process of a chemical reaction is often characterised by what is known as the transition state, which sets the path reactants must take to become products. The transition state is a structure that defines the key intermediate step, or bottleneck, in the path of least resistance for a given reaction.
However, it is becoming increasingly clear to scientists that reactions don’t always adhere to such easily defined routes. Over the last 15 years, they have been seeking to explain and observe a mechanism known as ‘roaming’, and while this is more complex, it is possible that it competes with the traditional, simple path in photodissociation reactions.
A roaming reaction can occur when there is a shallow plateau in the potential energy of the reactants. After the reaction has been initiated with the breaking of a bond, a much weaker interaction between the two fragments means they do not completely separate. The two fragments remain weakly connected and roam around one another at a distance of just a few Angstroms, exploring a wide range of geometries and configurations. This can end in one of two ways - either the two parties react to create new products, or they collide and gain enough energy to finally break apart.
Roaming has proven particularly difficult to observe and measure due to the ill-defined nature of the intermediate bond. Roaming partners can take on a wide range of structures, and their formation and deformation typically occur at femtosecond and nanosecond timescales. It is usually the distribution of energy in the final products that is used to show a roaming reaction has occurred. However, this does not explicitly reveal the nature and timescale of the intermediate bond or its ultimate fate.
Scientists from the University of Southampton, UCL and The Radboud University, working with the CLF’s Artemis facility have now presented a time-resolved observation of roaming reaction and specifically identified the roaming intermedate. To do this, they used a femtosecond extreme ultraviolet laser pulse to measure the time-resolved photoelectron spectra of acetaldehyde, excited at 4.7eV.
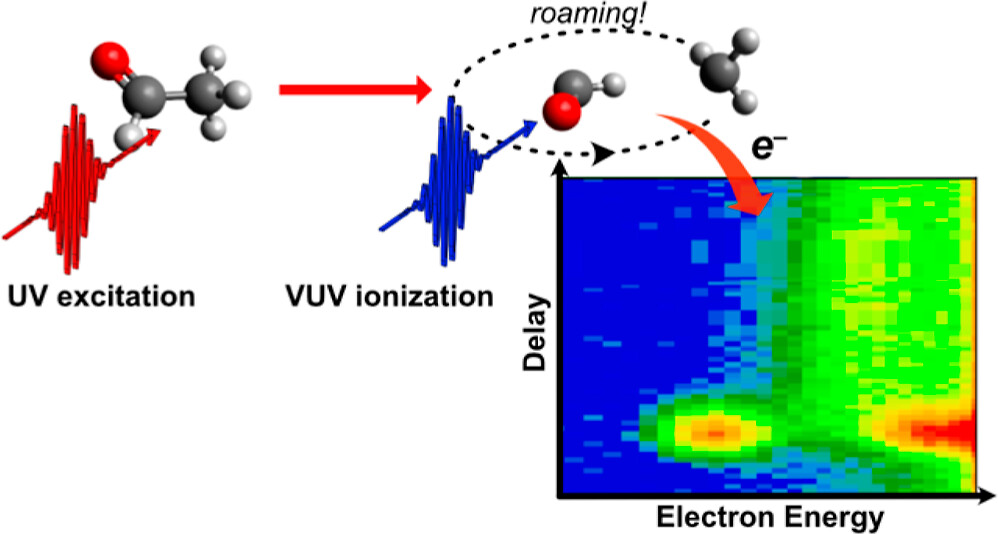
Image credit: Direct Observation of a Roaming Intermediate and Its Dynamics | Journal of the American Chemical Society (acs.org)
UV absorption breaks the carbon-carbon bond in acetaldehyde to form CH3 and HCO radicals. These radicals can then take the traditional reaction pathway, where they would quickly break apart, or they can experience roaming. In this instance the fragments roam at a distance of 3-4 Angstroms from one another before they either react to form CH4 and CO or break apart from one another. Both scenarios see the process last for much longer than the typical route.
While this channel has been previously studied in both theory and experiment, a group has yet to record the pathway of the intermediate bond until now. The advantage of the Artemis team’s photoelectron spectroscopy technique is that it is very sensitive to subtle changes in bonding and is high in energy. Through this method the group were able to capture spectral signatures for all reactants and products in the process, including the roaming intermediate bond. From analysis of this data and supporting quantum calculations they determined that the roaming reaction occurs on the electronic ground state, and the roaming intermediates break down on a timescale of 170 picoseconds. This is the first time this has been recorded through experiment and this detail may now be used to help quantify the importance of roaming pathways in photochemistry.
Read the full publication here: